
Salt Water Hydration? The Surprising Science of Electrolytes and Dehydration
Dehydration. You may have heard that if you’re working or exercising in hot temperatures or experiencing illness, you need to stay hydrated and that the simplest solution is to drink plenty of water or a sports drink such as Gatorade®. Read on for how salt water hydration works, and the dangers of dehydration.
First, to shed light on the issue and clear up common misconceptions, it is important to know why water is so important, what dehydration is, who’s at risk, and the stages of dehydration. We must also understand what electrolytes are and how they can prevent and treat dehydration when coupled with fluid replacement. Finally, there is the issue of sports drinks. Despite their popularity among certain groups, sports drinks have many limitations and often fall short in meeting many people’s hydration needs.
Water: The Most Important Nutrient
Water is the most important nutrient for your body. On average, the human body is 60 percent water by weight, depending on certain factors such as age, sex, and body weight. The average adult male is made up of 42 liters (or ~11 gallons) of water, while the average adult female is made up of 27.5 liters (~7.2 gallons) of water[1]. Water performs numerous important biological functions in the body. At the cellular level, it provides structural firmness [1] and makes up blood, lymph, gastric secretions, and urine. It also forms blood plasma, which transports oxygen, glucose, and amino acids to active muscle and tissue while carrying away carbon dioxide and lactic acid, which can impair muscle contractility and performance. Outside the cells, water helps lubricate our joints to allow bones to move freely against each other[2]. Additionally, the body uses water to adequately control its temperature through sweat. It’s estimated that endurance athletes can lose nearly 3 liters per hour, but even in moderately warm weather a significant amount of water is lost through sweat.
"Electrolytes are certain minerals (including calcium, chloride, magnesium, potassium, and sodium ions) essential to human health…and cannot be substituted by another or any other nutrient in the diet…"
What Are Electrolytes?
No discussion of dehydration would be complete without an explanation of electrolytes and their respective functions. Most people, when asked, are not sure what electrolytes are or why they’re so important in hydration. Electrolytes are certain minerals (including calcium, chloride, magnesium, potassium, and sodium ions) essential to human health, and are found naturally in salt water.
Hence, regardless of any other sugar or flavorings that may be added added to an electrolyte drink, the science begins with salt water hydration.
It is also important to know that the definition an essential mineral means that one electrolyte cannot be substituted by another or any other nutrient in the diet — your body will only accept that particular mineral.
Without all electrolytes, you could not move, think, or even live. Electrolytes are responsible for directing water and nutrients to the areas of the body where they are needed most and for maintaining optimal fluid balance inside the cells. Additionally, electrolytes help your muscles to contract and relax and assist in the transmission of nerve impulses from your nervous system to different body parts. Lack of electrolytes has been associated with muscle cramps or charley horses as some people call them.

Besides the functions listed above, studies show that repletion of one important electrolyte — magnesium — has a significant impact on athletic performance. Moderately trained athletes who took magnesium supplements showed decreased blood pressure, heart rate, and oxygen intake. Triathletes supplementing with extra magnesium demonstrated improved cycling, swimming, and running times [8]. Population studies consistently show that most adults do not get enough magnesium in their diet.
Dehydration Defined
Even a mild deficit of water can have a substantial impact on well-being, exercise performance, and attentiveness. Defined, dehydration is the loss of body water and important ions (blood salts like potassium and magnesium). This means your body doesn’t have as much water and electrolytes as it should have, which interferes with normal body processes. It’s easy to become dehydrated, and you don’t have to run a marathon to reach that state. Each day you lose approximately two to two-and-a-half cups (450 to 600 ml) of water just going about usual activities, so it is important to replace fluid lost throughout the day. Coffee, tea, and sodas are not an ideal choice because these beverages have a diuretic effect (i.e., they trigger water loss) and actually increase your daily fluid requirement. The current recommended dietary allowance of water for adults at rest under average conditions is 1 ml/kcal of energy expenditure [3]. For women, this amount would equal 2.2 l/day; for men it is 2.9 l/day [3].
Who’s At Risk for Dehydration?
Any individual can become dehydrated from the following conditions:
- Excessive sweating from endurance exercise, working outdoors, etc.
- Vomiting or diarrhea
- Fever
- Excessive urine output from uncontrolled diabetes or diuretic medications
- Infants, children, pregnant and breastfeeding women, those experiencing illness, and elderly adults have increased needs for water [3]. Infants and children, because of their smaller size and weight, can quickly become dangerously dehydrated if they’re experiencing vomiting, diarrhea, or fever and they refuse to eat or drink.
Excessive vomiting and diarrhea (lasting longer than 24 hours) is a cause for concern for anyone and is a risk factor for dehydration. Usually, the best way to treat this is to increase fluid and electrolyte intake to replace those lost. You can also add a salt water hydration solution to aid in obtaining crucial electrolytes, which can be sipped on every two or three minutes. If, however, a baby or adult is showing signs of dehydration (see below), one should seek medical attention immediately. Elderly adults can be at at increased risk because the thirst desire is reduced with age. It’s imperative that elderly adults (especially those who live in hot climates) drink plenty of fluids before they become thirsty.
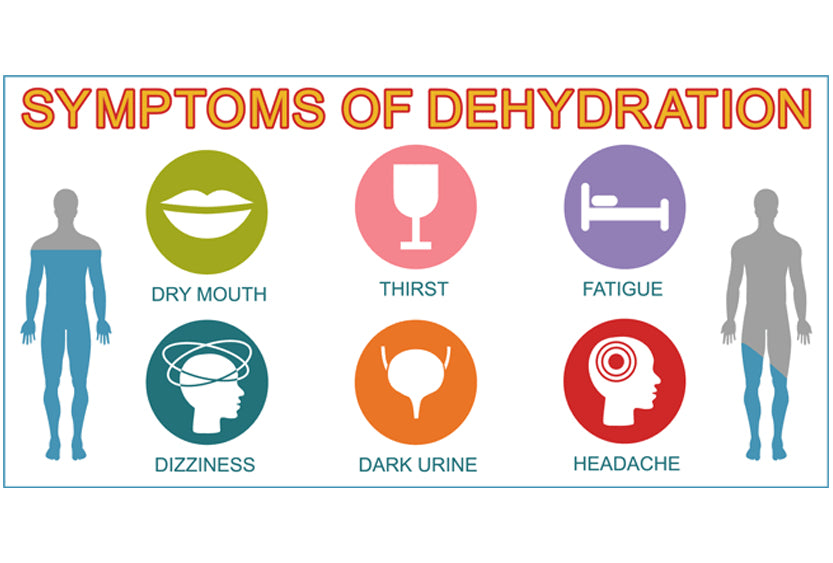
Three Classifications of Dehydration
There are three classifications of dehydration: mild, moderate, and severe. Each classification is based on the amount of fluid lost from the body that is not replaced.
Mild Dehydration – symptoms include:
- Dry lips and mouth
- Thirst
- Low urine output or urine appears dark yellow
Moderate Dehydration – symptoms include:
- Thirst
- Very dry mouth
- Sunken eyes
- Sunken fontanelles (the soft spots on an infant’s head)
- Tenting (skin doesn’t bounce back readily when pinched and lifted lightly)
- Low or no urine output
- Not producing tears
- At these signs, children under the age of 12 should see a physician immediately.
Severe Dehydration – symptoms include:
- All signs of moderate dehydration
- Rapid and weak pulse
- Cold hands and feet
- Rapid breathing
- Blue lips
- Lethargic, comatose, having seizures
- Severe dehydration requires immediate hospitalization.
How to Monitor Your Hydration Status
Thirst is a signal that your body needs fluid and electrolytes; however, it’s a poor indicator of your body’s needs because you can lose two percent of your body weight before you feel thirsty. A better way to gauge your hydration status is to monitor the output and color of your urine. A well-hydrated individual should void 1,000 to 1,500 ml/day, and urine color should be no darker than a pale yellow color [1]. If your urine is darker, it is a sign you are dehydrated and need to increase your fluid intake.
Dehydration’s Effect on Exercise Performance
Those who work or exercise in intense temperatures need to stay hydrated. Athletes should rely on urine output and color or checking their body weight both before and after exercise to gauge water loss. Ideally, athletes should replace approximately 1 liter of water per kg of weight lost (or ~2 cups/lb) [5].
Even mild water loss can significantly impede performance. For every one percent of body weight lost, blood volume decreases by 2.5 percent, muscle water decreases by one percent, and the body’s core temperature can increase 0.4 to 0.5° C [7]. Changes in blood volume during prolonged exercise impair the body’s ability to deliver oxygen and key nutrients to active muscles, organs, and glands and negatively affect thermoregulation (the body’s ability to regulate core body temperature) by diminishing the body’s ability to expel heat. Losses of three percent escalate these physiological changes and losses of nine to twelve percent are fatal [1,7].

Don’t Count on Sports Drinks to Stay Hydrated
Sports drinks are often touted as the ideal way to support hydration. Many claim to hydrate the body better than water and many contain a host of novel ingredients including vitamins, herbs, and caffeine to boost athletic performance. But are sports drinks more effective in hydrating the body than water?
Make no mistake — most sports drinks are no better than Kool-Aid with added sodium and, in some instances, potassium. Sports drinks are often loaded with sugar, and many athletes find them overwhelming when consumed during exercise or sporting events. Many commercial sports drinks are colored and flavored with chemicals and sweetened with high fructose corn syrup, a simple sugar that can cause fluctuations in blood sugar.
If you compared the grams of sugar (carbs) found in a typical 16-oz serving of several leading brands of sports drinks with the carb content found in your average Tootsie Roll, you would discover the following:
- Gatorade® contains 100 calories and 28 grams of carbs, which is equivalent to 13 Tootsie Rolls.
- Powerade® contains 34 grams of carbs, equivalent to 16 Tootsie Rolls.
- Endurox R-4 (Fruit Punch) contains 360 calories and 69 grams of carbs, equal to 33 Tootsie Rolls.
- Incidentally, a 16 oz.- serving of Kool-Aid* contains 32 grams of carbs, roughly equivalent to 15 Tootsie Rolls.
Common complaints with sports drinks include upset stomach, bloating, and a “mucusy, gagging sensation” in the back of the throat. Electrolytes—not sugar—support hydration to the cellular level, and sports drinks will have you maxing out on sugar before you’re adequately hydrated. The sugar content alone restricts the use of sports drinks for people with diabetes, which is highly telling.
Aside from the effect of sports drinks on blood-sugar levels, the long-term effects of the sweeteners, coloring agents, and other chemicals in sports drinks are not known; however, some research does raise questions. A 2005 study published in General Dentistry reported that some popular sports and energy drinks destroyed tooth enamel more severely than cola, finding that irreversible enamel damage was three to eleven times greater among the non-cola and sports beverages than cola-based drinks [9].
Another limitation of sports drinks is their electrolyte balance. Many claim to contain electrolytes to replace sweat loss, yet the primary electrolytes these beverages contain are sodium and potassium. That’s it. Most people already get too much sodium from food. The electrolyte content of Gatorade is 220 mg of sodium and 60 mg of potassium, based on a 16 oz. serving size. Powerade contains 110 mg of sodium and 60 mg of potassium. Gatorade Endurance, which claims to have five electrolytes, contains a whopping 400 mg of sodium and 180 mg of potassium. But what about the other electrolytes? Calcium and magnesium are mentioned; however, Endurance provides less than two percent of the Daily Value for these two critical electrolytes.
A balance of ALL electrolytes is necessary to maintain optimal hydration and endurance. You lose more than just sodium in sweat, you also lose other critical electrolytes like magnesium, and since most people don’t get enough magnesium, serious deficits can occur without balanced salt water hydration.
Salt Water Hydration: How To Prevent Dehydration
Plain water and sports drinks are not enough to meet your body’s hydration and electrolyte needs. Plain water (including bottled “mineral water”) doesn’t contain a substantial amount or the proper balance of the essential electrolytes you require to stay adequately hydrated and maintain optimum performance. As for sports drinks, the high-sugar content of most of these beverages can impair your hard-fought training and performance at the moment when it may matter the most. For these reasons, it is better to supplement your water with balanced electrolytes or sea salts, which naturally contain the multiple electrolytes your body needs. Salt water hydration solutions containing all the major electrolytes and no/low sugar are your best insurance policy.
References:
1 Taylor PN, Wolinsky, I., Klimis DJ (1999). Water in Exercise and Sport in Macroelements, Water, and Electrolytes, JA Driskell and Wolinsky I, Eds.,CRC Press, Boca Raton, FL: chap.5.
2 Christian JL and Greger JL (1994). In Nutrition for Living, 4th ed., Benjamin/Cummings, Redwood City, CA: chap.4.
3 National Research Council (1989). Water and Electrolytes, In Recommended Dietary Allowances, 10th ed., National Academy of Sciences, Washington, D.C., chap. 11.
4 Meletis, CM (2002). Dehydration: An Imbalance of Water and Electrolytes. Ogden, UT: By license to Mineral Resources International.
5 Clark, N (1997). In Nancy Clark’s Sports Nutrition Guidebook, 2nd ed., Human Kinetics, Champaign, IL, chap. 9.
6 Hultman E, Harris RC, Spriet LL. (1994). Work and exercise, In Modern Nutrition in Health and Disease, 8th ed.,Shilds ME, Olson JA, and Shike M., Eds., Lea & Febiger, Philadelphia, PA: chap. 42.
7 Wilmore JH and Costill DL (1994). In Physiology of Sport and Exercise, Human Kinetics, Champaign, IL:chap. 15.
8 Seelig, MS(2001). Human Needs for Magnesium are Not Met by Most People. Ogden, UT: Mineral Resources International.
9 von Fraunhofer AJ, Rogers MM. Effects of sports drinks and other beverages on dental enamel. General Dentistry 2005;53(1):308-312.
© 2020. All rights reserved.
The clinical studies and research articles cited in this article related to salt water hydration are for information purposes only and does not constitute an endorsement of Elete Electrolyte Add-In.
*Sample products compared were Gatorade Berry Citrus, Powerade Fruit Punch, and Kool-Aid Sugar-Sweetened Soft Drink, Grape Flavor.
**Gatorade, Powerade, and Kool-Aid are registered trademarks. Comparison based on 16-oz. serving.
This blog was sourced from elete.com


